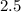Answer:
The ration of molar concentration is "2.5".
Step-by-step explanation:
The given values are:
Average density of salt water,
=
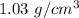
Net pressure,
=
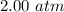
Increase in pressure,
=
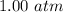
Now,
The under water pressure will be:
=
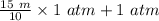
=
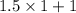
=
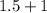
=
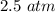
hence,
The ratio will be:
=
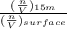
or,
=
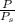
=
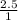
=