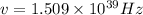Answer:
the frequency of photons
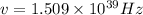
Step-by-step explanation:
Given: first ionization energy of 1000 kJ/mol.
No. of moles of sulfur = 1 mole
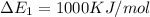
We know that plank's constant
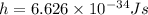
Let the frequency of photons be ν
Also we know that ΔE = hν
this implies ν = ΔE/h
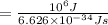
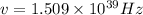
Hence, the frequency of photons