Answer:
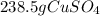
Step-by-step explanation:
Hello there!
In this case, by considering the given chemical reaction, it is possible for us to calculate the required grams of CuSO4 by considering its molar mass of 159.6 g/mol, the given molecules of NaBr, the Avogadro's number and the 1:2 mole ratio between these two in order to set up the following stoichiometric set up:
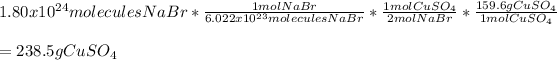
Best regards!