Answer:
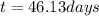
Step-by-step explanation:
Hello there!
In this case, since the radioactive decay is considered as first-order kinetic model, whereby the remaining mass of the radioactive material involves the initial one, the rate constant and elapsed time:
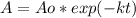
As we were not initially given the rate constant, we can use the half-life to calculate it as follows:
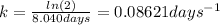
Thus, we can calculated the elapsed time for the given conditions to obtain:
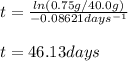
Regards!