Answer:
(a) η = 0.1742 = 17.42 %
(b) W = 31.64 W
(c) m = 0.58 kg
Step-by-step explanation:
(a)
The efficiency of a Carnot's engine is given as follows:
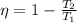
where,
η = efficiency = ?
T₁ = source temperature = 100°C + 273 = 373 K
T₂ = sink temperature = 35°C + 273 = 308 K
Therefore,

η = 0.1742 = 17.42 %
(b)
Another formula for the efficiency of Carnot's Engine is:
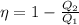
where,
Q₁ = Input heat rate
Q₂ = Heat rejected = 150 W
Therefore,
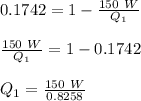
Q₁ = 181.64 W
Now, for the useful power output:
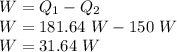
where,
W = Useful Output
W = 31.64 W
(c)
The heat required to condense steam i given as:
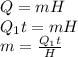
where,
m = mass of steam condensed = ?
t = time = 2 h = 7200 s
H = Latent heat of condensation of steam = 2260 KJ/kg = 2260000 J/kg
Therefore,
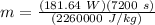
m = 0.58 kg