Answer: Concentration of the acid is 0.551 M.
Step-by-step explanation:
Given:
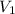 = 71.1 mL,
= 71.1 mL,
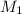 = 0.695 M
= 0.695 M
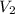 = 89.7 mL,
= 89.7 mL,
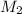 = ?
= ?
Formula used to calculate the concentration of acid is as follows.
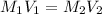
Substitute the values into above formula as follows.
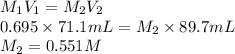
Thus, we can conclude that concentration of the acid is 0.551 M.