Answer:
Q= m
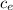 ΔT and Q = m L
ΔT and Q = m L
Step-by-step explanation:
For this graph of temperature vs energy (heating) we must use two relations
* for when there is no change of state
Q= m
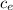 ΔT
ΔT
* for using there is change of state
Q = m L
the second expression is a consequence of the fact that all the energy supplied is used to change the state of the solid-liquid and liquid-gas system
the energy supplied is the sum of the energy in each interval
divide the system into intervals determined by the state change points
1) from T₀ = 70ºC to T_f = 327.4ºC, sample in solid-liquid state
c_e = 128 J / kg ºC
Q₁ = m c_e (T_f -To)
Q₁=1 128 (327.4 -70)
Q₁ = 3.29 10⁴ J
Q = Q₁ = 3.29 10⁴ J
2) when is it changing from solid to liquid
L = 2.45 10⁴ J / kg
Q2 = 1 2.45 10⁴
Q2 = 2.45 10⁴ J
Q = Q₁ + Q₂
Q = 5.74 10⁴ J
3) from to = 327.4ºC until T_f = 1725ºC, sample in liquid state
in the tables the specific heat of the solid and liquid state is the same
Q3 = m c_e (T_f -To)
Q3 = 1 128 (1725 -327.4)
Q3 = 1.79 10⁵ J
Q = Q₁ + Q₂ + Q₃
Q = (3.29 +2.45 + 17.9) 10⁴ J
Q = 23.64 10⁴ J
4) for when it is changing from the liquid state to the gaseous state
L_v = 8.70 10⁵ J / kg
Q₄ = m L_v
Q₄ = 1 8.70 10⁵
Q₄ = 8.70 10⁵ J
Q = Q₁ + Q₂ + Q₃ + Q₄
Q = (3.29 +5.74 + 17.9+ 87.0) 10⁴ J
Q = 110.64 10⁴ J
5) from To = 1725ºC to T_f = 2000ºC, sample in gaseous state
Q₅ = m c_e ΔT
Q₅ = 1 128 (2000 -1725)
Q₅ = 3.52 10⁴ J
Q = Q₁ + Q₂ + Q₃ + Q₄ + Q₅
Q = 114.16 104 J
the following table shows the points to be plotted
Energy (10⁴ J) Temperature (ºC)
0 70
3.29 327.4
5.74 327.4
23.64 1725
110.64 1725
114.16 2000
In the attachment we can see a graph of Temperature versus energy supplied