Answer:
Q = 4133.2 J
Step-by-step explanation:
Hello there!
In this case, since these calorimetry problems are based off the following heat equation involving heat (Q), mass (m), specific heat (C) and temperatures (T):
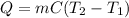
Thus, we plug in the given variables to obtain:
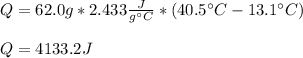
Regards!