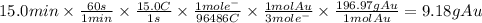Answer:
9.18g
Step-by-step explanation:
Step 1: Write the reduction half-reaction
Au³⁺(aq) + 3 e⁻ ⇒ Au(s)
Step 2: Calculate the mass of gold is produced when 15.0A of current are passed through a gold solution for 15.0min
We will use the following relationships:
- 1 mole of electrons has a charge of 96486 C (Faraday's constant).
- 1 mole of Au is produced when 3 moles of electrons circulate.
- The molar mass of Au is 196.97 g/mol.
The mass of gold produced is: