Answer: Molarity of the solution is
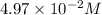 and water is the solvent.
and water is the solvent.
Step-by-step explanation:
Given: Mass of solute = 26.8 g
Volume = 4.00 L
Now, moles of copper (II) chloride (molar mass = 134.45 g/mol) are calculated as follows.
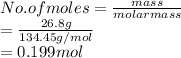
Molarity is the number of moles of a substance divided by volume of solution in liter.
Therefore, molarity of given solution is calculated as follows.
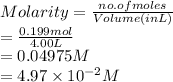
Solvent is defined as a component which is present in higher amount in a solution. Generally, a solvent is present in liquid state but it can also be a solid or gas.
In the given solution, copper (II) chloride is dissolved in water so copper (II) chloride is the solute and water is the solvent.
Thus, we can conclude that molarity of the solution is
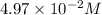 and water is the solvent.
and water is the solvent.