Answer:
The value becomes 0.0821 L-atm/K⁻¹-mol⁻¹.
Step-by-step explanation:
The value of gas constant is 0.0821.
We know that, the ideal gas law is as follows :
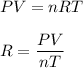
Where
P is pressure
V is volume
T is temperature
In order to use the Ideal Gas Constant of 0.0821, the units are follows :
Volume = Litre (L)
Pressure = atm
Amount = mol⁻¹
Temperature = K⁻¹
So, the value becomes 0.0821 L-atm/K⁻¹-mol⁻¹.