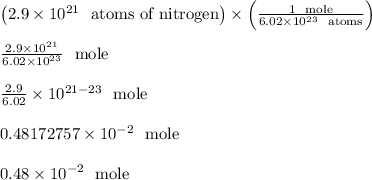
Answer: Approximately
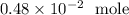
============================================================
Step-by-step explanation:
There are roughly 6.02 * 10^23 atoms in one mole of any element. So we have 6.02 * 10^23 atoms in one mole of nitrogen.
We can then do the following conversion: