Answer: D) 1.6 mol
Step-by-step explanation:
This is a stoichiometry problem that uses the ratios of the coefficients of the chemical equation. We see from the equation that 2 moles of C4H10 produces 10 moles of H20.
Now if we had 8 moles of H20 then we must have started with 1.6 moles of C4H10. This is because
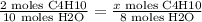
This gives us x = 1.6 moles of C4H10