Answer: The concentration of
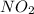 in the given equilibrium mixture is 0.0545 M.
in the given equilibrium mixture is 0.0545 M.
Step-by-step explanation:
The ratio of concentration of products and reactants raised to the power of their coefficients is called equilibrium constant. The symbol used to denote equilibrium constant is
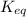 .
.
As the given reaction equation is as follows.
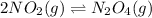
The expression for equilibrium constant of this reaction is as follows.
![K_(eq) = ([N_(2)O_(4)])/([NO_(2)]^(2))](https://img.qammunity.org/2022/formulas/chemistry/college/o4vj4e3xi8grkgcpxv47y8adzwynrqgkr2.png)
Now, substitute the given values into above formula as follows.
![K_(eq) = ([N_(2)O_(4)])/([NO_(2)]^(2))\\67.3 = ((0.2))/([NO_(2)]^(2))\\So, [NO] = \sqrt{(0.2)/(67.3)}\\= 0.0545 M](https://img.qammunity.org/2022/formulas/chemistry/college/es2qbn6pvm9r8kakjmywnuzhwjg53jv1xl.png)
Thus, we can conclude that the concentration of
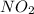 in the given equilibrium mixture is 0.0545 M.
in the given equilibrium mixture is 0.0545 M.