Answer: The volume of nitrogen gas at STP is 44.8 L.
Step-by-step explanation:
The number of moles is defined as the ratio of the mass of a substance to its molar mass. The equation for it follows:
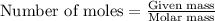 ...(1)
...(1)
We are given:
Given mass of
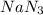 = 130.0 g
= 130.0 g
Molar mass of
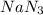 = 65.01 g/mol
= 65.01 g/mol
Using equation 1:
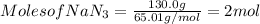
For the given chemical equation:
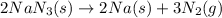
By the stoichiometry of the reaction:
2 moles of
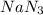 produces 3 moles of nitrogen gas
produces 3 moles of nitrogen gas
At STP:
1 mole of a gas occupies 22.4 L of volume
So, 2 moles of nitrogen gas will occupy
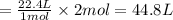 of volume
of volume
Hence, the volume of nitrogen gas at STP is 44.8 L.