Answer: 1). The temperature of this gas is 1032.88 K.
2). There are 21.48 moles of gas exist at a pressure of 14.5 atm, a volume of 45 liters, and a temperature of
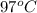 .
.
Step-by-step explanation:
1). Given: No. of moles = 6 moles
Pressure = 10.6 atm
Volume = 48 L
Formula used to calculate temperature is as follows.
PV = nRT
where,
P = pressure
V = volume
n = no. of moles
R = gas constant = 0.0821 atm
T = temperature
Substitute the values into above formula as follows.
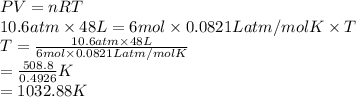
Hence, temperature of this gas is 1032.88 K.
2). Given: Pressure = 14.5 atm
Volume = 45 L
Temperature =
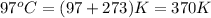
Formula used to calculate number of moles is as follows.
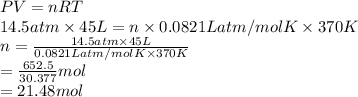
Hence, there are 21.48 moles of gas exist at a pressure of 14.5 atm, a volume of 45 liters, and a temperature of
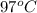 .
.