Answer:

Step-by-step explanation:
We are asked to convert atoms to moles.
1. Avogadro's Number
We know that 1 mole of any substance contains the same number of particles (atoms, molecules, formula units etc.) This is Avogadro's Number: 6.022*10²³. For this question, the particles are atoms of gold (Au).
2. Convert Atoms to Moles
Let's set up a proportion using this information.
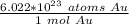
Since we are solving for the moles in 9.75 * 10²⁴ atoms, we multiply by that number.
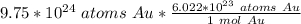
Flip the proportion. It will be equivalent, but the units of "atoms Au" can cancel.
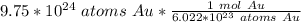
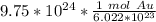
Condense the problem into 1 fraction.
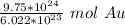
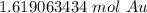
3. Round
The original measurement (9.75) has 3 significant figures. Our answer must have the same. For the number we found, that is the hundredth place.
The 9 in the thousandth place tells us to round the 1 to a 2 in the hundredth place.
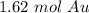
9.75 * 10²⁴ atoms of gold is approximately equal to 1.62 moles of gold.