Answer: The value of
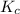 for the given reaction is
for the given reaction is
![K_(c) = ([HI]^(2))/([H_(2)][I_(2)])](https://img.qammunity.org/2022/formulas/chemistry/college/2subit6zrli6jhpn9r6wbbtjuhs4cls365.png) .
.
Step-by-step explanation:
The ratio of concentration of products and reactants raised to the power of their concentration is called equilibrium constant.
It is denoted by the symbol
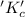 .
.
For example,
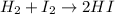
The expression for
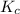 of this reaction is as follows.
of this reaction is as follows.
![K_(c) = ([HI]^(2))/([H_(2)][I_(2)])](https://img.qammunity.org/2022/formulas/chemistry/college/2subit6zrli6jhpn9r6wbbtjuhs4cls365.png)
So, by putting the respective concentrations of the species involved in the reaction it is possible to determine the value of
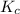 for the given reaction.
for the given reaction.
Thus, we can conclude that the value of
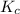 for the given reaction is
for the given reaction is
![K_(c) = ([HI]^(2))/([H_(2)][I_(2)])](https://img.qammunity.org/2022/formulas/chemistry/college/2subit6zrli6jhpn9r6wbbtjuhs4cls365.png) .
.