Answer:
Step-by-step explanation:
If we want to convert from moles to grams, we must use the molar mass. This values tells us the mass of 1 mole of a substance. They can be found on the Periodic Table; they are equivalent to the atomic masses, but the units are grams per mole (g/mol) instead of atomic mass units (amu).
1. Molar Mass
We have the compound ammonia or NH₃. Look up the molar masses for the individual elements.
- Nitrogen (N): 14.007 g/mol
- Hydrogen (H): 1.008 g/mol
Check for subscripts. There is a subscript of 3 after hydrogen. This means there are 3 atoms of hydrogen in 1 molecule of ammonia. We should multiply hydrogen's molar mass by 3.
- H₃= 1.008 * 3=3.024 g/mol
Add nitrogen's molar mass.
- NH₃: 14.007 + 3.024=17.031 g/mol
2. Convert Moles to Grams
Now, use the molar mass as a ratio.
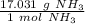
Since we are trying to find the mass of 1.204 moles, we multiply by that value.
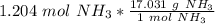
The units of "moles of ammonia" cancel.
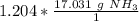
The denominator of 1 can be ignored.
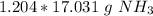
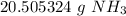
3. Round
The original measurement of moles has 4 significant figures, so our answer must have the same. For the number we calculated, that is the hundredth place.
The 5 in the thousandth place tells us to round to 0 to a 1 in the hundredth place.
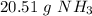
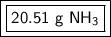
1.204 moles of ammonia is equal to 20.51 grams of ammonia.