Answer:
23.12 atm
Step-by-step explanation:
First, add together the moles of the two samples:
5.25 moles + 4.20 moles = 9.45 moles
273 + 25 = 298 K for the temperature
volume is 10.0 L
Since we have moles now, we have to rearrange our ideal law equation to solve for pressure:
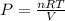
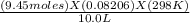
9.45 X .08206 X 298 all divided by 10.0 = 23.09202 atm (or 23.12)