Answer:

Step-by-step explanation:
At standard temperature and pressure (STP), 1 mole of any gas has a volume of 22.4 liters. This applies to oxygen, so let's create a proportion.
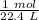
Since we are trying to find the moles in 11.2 liters, we should multiply by that number.
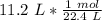
The units of liters cancel.
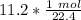
Condense into 1 fraction.
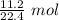
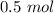
At STP, 11.2 liters of oxygen gas is equal to 0.5 moles of oxygen gas.