Answer: The solubility product of AgCl is
 .
.
Step-by-step explanation:
The reaction equation is as follows.
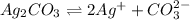
Let us assume the concentration of
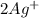 is 2S and concentration of
is 2S and concentration of
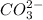 is S. Hence, the expression for
is S. Hence, the expression for
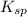 of this reaction is as follows.
of this reaction is as follows.
![K_(sp) = [Ag^(+)]^(2)[CO^(2-)_(3)]\\8.2 * 10^(-12) = (2S)^(2)(S)\\8.2 * 10^(-2) = 4S^(3)\\S = 1.27 * 10^(-4)](https://img.qammunity.org/2022/formulas/chemistry/college/dy663axovpnrq8nrwykzbu5eda288m1dy3.png)
This means that
![[Ag^(+)]](https://img.qammunity.org/2022/formulas/chemistry/college/4lzuy94965tgcammf3he3ydsfhehvsgfxl.png) is
is
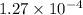 . Now, the concentration of
. Now, the concentration of
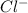 is calculated as follows.
is calculated as follows.
![[Cl^(-)] = (mass)/(molar mass)\\= (0.003 g)/(35.5 g/mol)\\= 8.45 * 10^(-5) M](https://img.qammunity.org/2022/formulas/chemistry/college/4d3hh0t11t45eacpj2ebno062xnn9asxi8.png)
Hence,
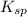 for AgCl is calculated as follows.
for AgCl is calculated as follows.
![K_(sp) = [Ag^(+)] * [Cl^(-)]\\= 1.27 * 10^(-4) * 8.45 * 10^(-5)\\= 10.73 * 10^(-9)](https://img.qammunity.org/2022/formulas/chemistry/college/yjgn7bsjaw1toazsw9cnvhb4nz564rf850.png)
Thus, we can conclude that solubility product of AgCl is
 .
.