Answer:
structural arrangements
_______________________________________
properties of daimond:
appearance: transparent
hardness: very hard
thermal conductivity :very poor
electric conductivity: poor
density:
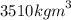
uses: jewellery and drilling
_______________________________________
properties of graphite:
appearance: black shiny
hardness: soft ,slippery to touch
thermal conductivity : moderate
electric conductivity: good
density:
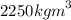
uses:dry cell, electric arc, pencil lead, lubricant
_______________________________________
How Diamond and Graphite are chemically identical?
- On heating diamond or graphite in the air, they burn completely to form carbon dioxide.
- - Equal quantities of diamond and graphite when burned, produce exactly the same amount of carbon dioxide.
_______________________________________
Why the physical properties of diamond and graphite are so different?
Due to the difference in the arrangement of carbon atoms in diamond and graphite
_______________________________________
hope it helps you<3