The question is: Calculate the molar fractions of the components of a solution made up of: 250 g of water, 50 g of acetic acid and 25 g of sodium chloride.
Answer: The mole fraction of water, acetic acid and sodium chloride is 0.916, 0.054, and 0.028.
Step-by-step explanation:
As number of moles is the mass of substance divided by its molar mass.
Therefore, moles of given species are calculated as follows.
The number of moles of water (molar mass = 18 g/mol):
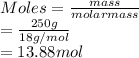
The number of moles of acetic acid (molar mass = 60.052 g/mol):
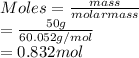
The number of moles of sodium chloride (molar mass = 58.44 g/mol):
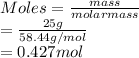
Therefore, mole fraction of each component is calculated as follows.
Mole fraction of water:
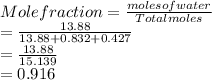
Mole fraction of acetic acid:
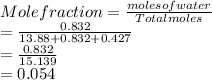
Mole fraction of NaCl:
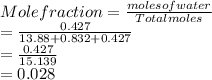
Thus, we can conclude that mole fraction of water, acetic acid and sodium chloride is 0.916, 0.054, and 0.028.