Answer: The new temperature is 4768 K.
Step-by-step explanation:
Given:
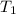 = 298 K,
= 298 K,
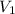 = V,
= V,
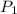 = P
= P
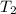 = ?,
= ?,
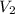 = 4V,
= 4V,
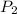 = 4P
= 4P
Using the combined gas law formula as follows.
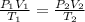
Substitute the values into above formula as follows.
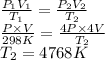
Thus, we can conclude that the new temperature is 4768 K.