Given :
Molarity of acetic acid solution, M = 0.10 M.
pKa of acetic acid, pKa = 4.75 .
To Find :
Percentage dissociation of 0.10 M solution of acetic acid.
Solution :
We know,
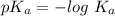
Taking antilog both side, we get :
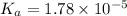
Since, acetic acid has 1 hydrogen atom to loose , so it is a monoprotic acid.
Now, percentage dissociation of monoprotic acid is given by :
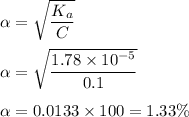
Hence, this is the required solution.