Given :
Mass of gold ( Au ) is 212 gram.
To Find :
Atoms of gold present in 212 gram of gold.
Solution :
Molecular mass of gold is 197 gram.
So, number of moles of gold in 212 gram is :
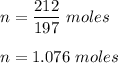
Now, we know 1 mole of any element contains
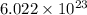 atoms.
atoms.
So, number of atoms present in 1.076 moles are :
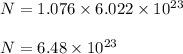
Hence, this is the required solution.