Answer:
Step-by-step explanation:
Hello there!
In this case, since this concentration of hydronium ions is given, we infer we may calculate that of the hydroxide ions, pH and pOH because no question was specifically given. That is why we proceed as follows:
- pH: Here, we use this concentration to calculate the pH according to the negative logarithm:
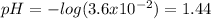
Which means that the solution is acidic as the pH is less than 7.
- pOH: since the pH added to the pOH is 14, we can calculate the latter as follows:
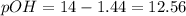
- Hydroxide concentration: Given the pOH, we apply the antilogarithm to calculate such concentration as shown below:
![[OH^-]=10^(-pOH)=10^(12.56)=2.75x10^(-13)M](https://img.qammunity.org/2022/formulas/chemistry/college/7yn06n0gf28xnobkd8v8loluvr3rqsegx6.png)
Keep in mind, this is an arbitrary solution because no question was provided.
Regards!