Answer: There are 0.00054 moles of silane gas (SiH4) are present in 8.68 mL measured at 18 0C and 1.50 atm.
Step-by-step explanation:
Given: Volume = 8.68 mL (1 mL = 0.001 L) =
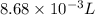 ,
,
Temperature =
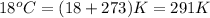 ,
,
Pressure = 1.50 atm
The ideal gas formula is as follows.
PV = nRT
where,
P = pressure
V = volume
n = no. of moles
R = gas constant = 0.0821 L atm/mol K
T = temperature
Substitute the values into above formula as follows.
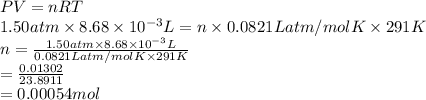
Thus, we can conclude that there are 0.00054 moles of silane gas (SiH4) are present in 8.68 mL measured at 18 0C and 1.50 atm.