Answer:
![[CO]=[Cl_2]=0.01436M](https://img.qammunity.org/2022/formulas/chemistry/college/8xrp5mq9n6l71eig2gdm0mdkcb9pxjzqqw.png)
![[COCl_2]=0.00064M](https://img.qammunity.org/2022/formulas/chemistry/college/6tuw9ieefjo9cu9eu6mph2b4v53hcw8vpv.png)
Step-by-step explanation:
Hello there!
In this case, according to the given chemical reaction at equilibrium, we can set up the equilibrium expression as follows:
![K=([CO][Cl_2])/([COCl_2])](https://img.qammunity.org/2022/formulas/chemistry/college/5uczzrqdtfzn0xe26pmjsfbcx7dr5rozm5.png)
Which can be written in terms of x, according to the ICE table:
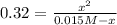
Thus, we solve for x to obtain that it has a value of 0.01436 M and therefore, the concentrations at equilibrium turn out to be:
![[CO]=[Cl_2]=0.01436M](https://img.qammunity.org/2022/formulas/chemistry/college/8xrp5mq9n6l71eig2gdm0mdkcb9pxjzqqw.png)
![[COCl_2]=0.015M-0.01436M=0.00064M](https://img.qammunity.org/2022/formulas/chemistry/college/wmzbccfbpaz7jnm75o9iag10dhp4j00f0h.png)
Regards!