Answer:
0.631 grams is the theoretical yield of solid copper (Cu) that can be recovered at the end of the experiment
Step-by-step explanation:
The concentration of the solution is given by :
![[C]=\frac{\text{Moles of compound}}{\text{Volume of solution in Liters}}](https://img.qammunity.org/2022/formulas/chemistry/college/sy02jhkifjhe22yhgydl1v7m3w26fdpq11.png)
We have:
Concentration of copper (II) nitrate solution =
![[Cu(NO_3)_2]=2.41 M](https://img.qammunity.org/2022/formulas/chemistry/college/4kkvlypasl223ud6dhnqoagnmyf72zoxe4.png)
The volume of solution = 4.12 mL
1 mL= 0.001 L
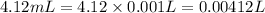
Moles of copper (II) nitrate in solution = n
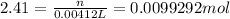
Moles of copper (II) nitrate in solution = 0.0099292 mol
1 Mole of copper(II) nitrate has 1 mole of copper then 0.0099292 moles of copper(II) nitrate will have :
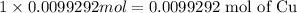
Mass of 0.0099292 moles of copper:
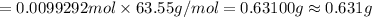
This mass of copper present in the solution is the theoretical mass of copper present in the given copper(II) nitrate solution.
0.631 grams is the theoretical yield of solid copper (Cu) that can be recovered at the end of the experiment