Answer:
A) λ = 4.88 10² nm, B) λ = 4.08 10² nm
Step-by-step explanation:
The spectrum of hydrogen is correctly explained by the Bohr model, where the energy of each level is
Eₙ = -13.606 /n² [eV]
the transition generally occurs from a given level to a lower state nf <no, so a transition is
ΔE = E_f -Eₙ = -13,606 (
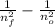 )
)
to find the wavelength let's use the planck relation
ΔE = h f
the speed of light is
c = λ f
we substitute
ΔE = h c /λ
λ =
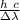
let's apply this equation to our case
the Balmer series has as final state the level n_f = 2
A) initial state n₀ = 4, final state n_f = 2
ΔE = -13.606 (
 )
)
ΔE = 2.55 eV
let's reduce to SI units
ΔE = 2.55 eV (1.6 10⁻¹⁹ J / 1 eV) = 4.08 10⁻¹⁹ J
we calculate
λ = 6.63 10⁻³⁴ 3 10⁸ / 4.08 10⁻¹⁹
λ = 4.875 10⁻⁻⁷ m
we reduce to nm
λ = 4.875 10⁻⁷ m (10⁹ nm / 1m)
λ = 487.5 nm
we reduce to three significant figures
λ = 4.88 10² nm
B) initial state n₀ = 5
ΔE = -13,606 (
 )
)
ΔE = 2,857 eV
we repeat the process of the previous point
ΔE= 2,857 1.6 10⁺¹⁹ = 4.286 10⁻¹⁹J
we look for the wavelength
λ = 6.63 10⁻³⁴ 3 10⁸ / 4.88 10⁻¹⁹
λ = 4.0758 10⁻⁷ m
we reduce to nm
λ = 4.0758 10² nm
ignificant numbers
λ = 4.08 10² nm