Answer: The volume of 0.640 grams of
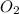 gas at Standard Temperature and Pressure (STP) is 0.449 L.
gas at Standard Temperature and Pressure (STP) is 0.449 L.
Step-by-step explanation:
Given: Mass of
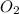 gas = 0.640 g
gas = 0.640 g
Pressure = 1.0 atm
Temperature = 273 K
As number of moles is the mass of substance divided by its molar mass.
So, moles of
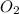 (molar mass = 32.0 g/mol) is as follows.
(molar mass = 32.0 g/mol) is as follows.
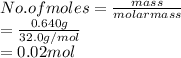
Now, ideal gas equation is used to calculate the volume as follows.
PV = nRT
where,
P = pressure
V = volume
n = no. of moles
R = gas constant = 0.0821 L atm/mol K
T = temperature
Substitute the values into above formula as follows.
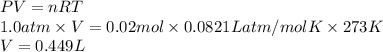
Thus, we can conclude that the volume of 0.640 grams of
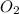 gas at Standard Temperature and Pressure (STP) is 0.449 L.
gas at Standard Temperature and Pressure (STP) is 0.449 L.