Answer:
3.1x10²³
Step-by-step explanation:
I'm going to answer the question assuming the number of molecules of FeCl₃ is asked, rather than the number of atoms.
- Fe₂O₃ + 6HCl → 2FeCl₃ + 6H₂O
First we convert 9.3x10²³ atoms of HCl into moles of HCl, using Avogadro's number:
- 9.3x10²³ atoms ÷ 6.023x10²³ atoms/mol = 1.54 mol HCl
Then we convert 1.54 moles of HCl into moles of FeCl₃, using the stoichiometric coefficients of the reaction:
- 1.54 mol HCl *
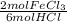 = 0.51 mol FeCl₃
= 0.51 mol FeCl₃
Finally we calculate how many FeCl₃ molecules are there in 0.51 moles:
- 0.51 mol * 6.023x10²³ molecules/mol = 3.4x10²³ molecules
The closest option is 3.1x10²³.
The number of atoms produced is four times the number of FeCl₃ molecules produced.