Answer:
The new volume will be 3.67 L.
Step-by-step explanation:
As the volume increases, the gas particles (atoms or molecules) take longer to reach the walls of the container and therefore collide with them fewer times per unit of time. This means that the pressure will be lower because it represents the frequency of collisions of the gas against the walls. In this way pressure and volume are related, determining Boyle's law which says:
"The volume occupied by a certain gaseous mass at constant temperature is inversely proportional to pressure"
Boyle's law is expressed mathematically as:
P*V=k
Now it is possible to assume that you have a certain volume of gas V1 that is at a pressure P1 at the beginning of the experiment. If you vary the volume of gas to a new value V2, then the pressure will change to P2, and it will be fulfilled:
P1 * V1 = P2 * V2
In this case:
- P1= 1.85 atm
- V1= 4.64 L
- P2= 2.34 atm
- V2= ?
Replacing:
1.85 atm* 4.64 L= 2.34 atm* V2
Solving:
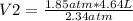
V2= 3.67 L
The new volume will be 3.67 L.