Question
A sample of methane collected when the temp was 30 C and 760mmHg measures 398 mL. What would be the volume of the sample at -5 C and 616 mmHg pressure
Answer:
434.32mL
Step-by-step explanation:
Using the combined gas law:
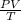 = k
= k
Where;
P = Pressure
V = Volume
T = Temperature
k = constant.
It can be deduced that:
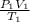 =
=
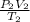 = k ---------------------(i)
= k ---------------------(i)
Where:
P₁ and P₂ are the initial and final pressures of the given gas
V₁ and V₂ are the initial and final volumes of the given gas
T₁ and T₂ are the initial and final temperatures of the gas.
From the question:
the gas is methane
P₁ = 760mmHg
P₂ = 616mmHg
V₁ = 398mL
V₂ = ?
T₁ = 30°C = (30 +273)K = 303K
T₂ = -5°C = (-5 +273)K = 268K
Substitute these values into equation (i) as follows;
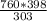 =
=
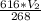
Solve for V₂
V₂ =
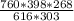
V₂ = 434.32mL
Therefore, the volume of the sample at -5C and 616mmHg pressure is 434.32mL