Answer: The volume is 57.52 mL if the temperature if raised to 343K.
Step-by-step explanation:
Given:
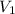 = 27 mL,
= 27 mL,
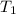 = 161 K
= 161 K
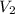 = ?,
= ?,
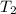 = 343 K
= 343 K
According to Charles law, at constant pressure the volume of an ideal gas is directly proportional to temperature.
Formula used is as follows.
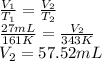
Thus, we can conclude that volume is 57.52 mL if the temperature if raised to 343K.