Answer:
M = 33.6M .
Step-by-step explanation:
Hello there!
In this case, according to the definition of molarity which is computed by dividing the moles of solute by the liters of solution, it is possible to realize that the moles of the solute are 12.6 mol and the liters of solution are 375/1000= 0.375 L and therefore the molarity of the solution can be calculated as follows:
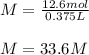
Which has molar units, M.
Regards!