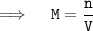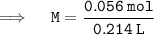Ⲁⲛ⳽ⲱⲉⲅ:
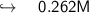
Ⲋⲟⳑⳙⲧⳕⲟⲛ :
Molarity is used to measure the concentration of a solution , so it is also as molar concentration. It is denoted as M or Mol/L
We are given that :
- Weight of
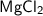 = 5.34g
= 5.34g - Volume of solution = 214 ml , or 0.214 L
The molar mass of magnesium chloride (
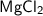 ) is 95.21 g / mol
) is 95.21 g / mol
We can calculate the molarity of the solution by dividing the number of moles of solute by volume of solvent in liter ,i.e:
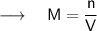 ㅤㅤㅤ⸻( 1 )
ㅤㅤㅤ⸻( 1 )
Where,
- M = molarity
- n = number of moles
- V = Volume
We can calculate the number of moles by dividing the actual mass by its molar mass ,i.e:
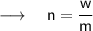 ㅤㅤㅤ⸻ ( 2 )
ㅤㅤㅤ⸻ ( 2 )
Where,
- n = number of moles
- m = molar mass
- w = actual mass
Therefore,
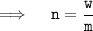
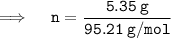

Putting the values in equation ( 1 ):