Solution :
Compound Ksp
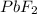
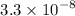
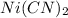
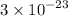
FeS
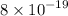
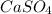
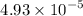
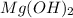
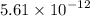
Ksp of
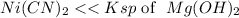 and both compounds dissociate the same way. Hence
and both compounds dissociate the same way. Hence
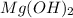 is more soluble than
is more soluble than
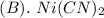
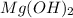 is less soluble than
is less soluble than
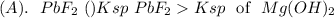
It is not possible to determine CD -
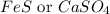 is more or less soluble than
is more or less soluble than
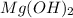 as though they have a different Ksp values their molecular dissociation is also different and they may have a close solubility values.
as though they have a different Ksp values their molecular dissociation is also different and they may have a close solubility values.
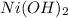 can be directly compared with PbS,
can be directly compared with PbS,
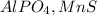
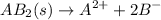
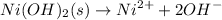
100
1-s s 2s
Ksp =
![[A2+][B-]^2 = s * (2s)^2 = 4s^3](https://img.qammunity.org/2022/formulas/chemistry/college/or8e4xpiyvdmg4kxopklsx566vwalyccmr.png)
Hence they can be directly compared by Ksp values, smaller the Ksp, smaller the solubility.
For Silver Chloride
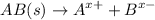
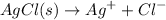
1 0 0
1 - s s s
Ksp
![$=[A^(x+)][B^(x-)]=s * s = s^2$](https://img.qammunity.org/2022/formulas/chemistry/college/qn1kpsyfi2vnoqe4nudmc9kgue152lx65o.png)
Hence, they can be directly compared by Ksp values, smaller the Ksp, smaller the solubility.