Answer:
B. -2
Step-by-step explanation:
The total charge on an atom is the sum of all individual charges present in it. Therefore, the total charge on this atom is given by the following formula:
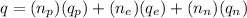
where,
q = total charge on atom = ?
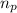 = no. of protons in the atom = 15
= no. of protons in the atom = 15
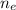 = no. of electrons in the atom = 17
= no. of electrons in the atom = 17
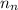 = no. of neutrons in the atom = 12
= no. of neutrons in the atom = 12
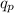 = charge on proton = +1
= charge on proton = +1
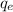 = charge on electron = -1
= charge on electron = -1
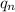 = charge on neutron = 0
= charge on neutron = 0
Therefore,
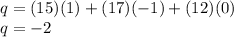
Hence the correct option is:
B. -2