Answer:
The half-life of the substance is about 288 days.
Explanation:
The exponential decay function:
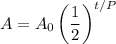
Can determine the amount A of a radioactive substance present at time t. A₀ represents the initial amount and P is the half-life of the substance.
We are given that a substance loses 70% of its radioactivity in 500 days, and we want to determine the period of the half-life.
In other words, we want to determine P.
Since the substance has lost 70% of its radioactivity, it will have only 30% of its original amount. This occured in 500 days. Therefore, A = 0.3A₀ when t = 500 (days). Substitute:
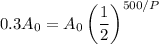
Divide both sides by A₀:
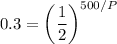
We can take the natural log of both sides:
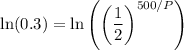
Using logarithmic properties:
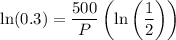
So:
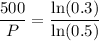
Take the reciprocal of both sides:
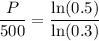
Use a calculator:
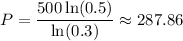
The half-life of the substance is about 288 days.