Answer: 1).
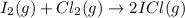 has
has
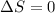 .
.
2).
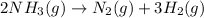 has
has
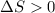 .
.
3).
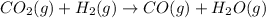 has
has
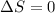 .
.
4).
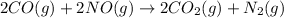 has
has
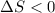 .
.
5).
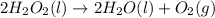 has
has
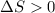 .
.
Step-by-step explanation:
Entropy is the increase in degree of randomness of a substance. When a solid is converted into liquid then disorderness among the molecules of a substance increases.
As a result, entropy increases when a solid state converts into a liquid state.
Similarly, when a liquid state converts into gaseous state then molecules move more randomly from one place to another. Hence, entropy further increases when solid or liquid state of substance changes into gaseous state.
1).
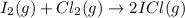
This shows that 2 gaseous reactants are giving 2 gaseous product. So, entropy will remain the same. Hence,
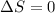 for this reaction.
for this reaction.
2).
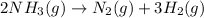
Here, 2 moles of ammonia gas is giving 4 moles of gaseous products. This means that number of gaseous moles are increasing. So, entropy will increase and therefore,
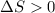 .
.
3).
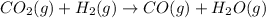
Here, 2 moles of gaseous reactants are giving 2 moles of gaseous products . So, there is no increase in number of gaseous moles therefore,
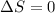 .
.
4).
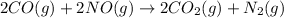
Here, total 4 moles of gaseous reactants are giving total 3 moles of gaseous products. This means a decrease in number of moles is taking place. Hence, entropy is decreasing and therefore,
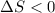 .
.
5).
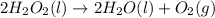
Here, 2 moles of reactants are giving total 3 moles of products. This means that an increase in number of moles is taking place. So, entropy will increase therefore,
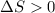 .
.