Answer:
25 grams of Mg(OH)2 will be produced by 14.424 gram of Mg3N2
Step-by-step explanation:
The balanced equation is
Mg3N2 + 6H2O -> 3Mg(OH)2 + 2NH3
Molecular weight of magnesium nitride = 100.9494 g/mol
Molecular weight of magnesium hydroxide = 58.3197 g/mol
one mole of Mg3N2 produces three moles of 3Mg(OH)2
100.9494 g/mol of Mg3N2 produces 3* 58.3197 g/mol of Mg(OH)2
1 gram of Mg3N2 produces
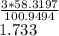 grams of Mg(OH)2
grams of Mg(OH)2
Or 1.733 grams of Mg(OH)2 will be produced by 1 gram of Mg3N2
25 grams of Mg(OH)2 will be produced by 14.424 gram of Mg3N2