Answer:
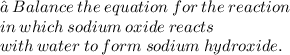
⇒ We have Na2O + H2O --> NaOH. We have 2 sodiums and 2 oxygens and 2 hydrogens on the left side, but only one of each on the right side.
Sodium Oxide + Water → Sodium Hydroxide
⇒ Na2O + H2O → 2NaOH .
Sodium oxide is used in ceramics and glasses. Sodium oxide reacts exothermically with cold water to produce sodium hydroxide solution.