Answer:
80.92, Krypton
Step-by-step explanation:
What is effusion?
• It is a process where gas escapes through a pinhole (a very small hole) into a region of low pressure or vacuum
Graham's law of effusion of gas
• states that at a given constant temperature and pressure, the rate of effusion of gases is inversely proportional to the square root of their molar masses
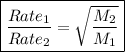
Calculations
Nitrogen exist as N₂ at room temperature, thus its molar mass is 2(14)= 28.
Let the rate and molar mass of unknown gas be Rate₂ and M₂ respectively.
Since N₂ effuses 1.7 times as fast as the unknown gas,
Rate₁= 1.7(Rate₂)
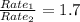
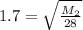
Square both sides:
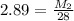
Multiply both sides by 28:
2.89(28)= M₂
M₂= 80.92
Identity of gas
The molar mass of 80.92 lies between Bromine and Krypton. However since Bromine exist as Br₂, the value of it's molar mass would be 159.8 instead. Hence, Bromine is eliminated.
If the gas is a diatomic element, the atomic weight is 80.92 ÷2= 40.46. Thus, we are now considering if Argon could be its identity. However, Argon is a noble gas and will not exist as a diatomic element. Argon is therefore eliminated too.
Thus based on the above reasoning, its probable identity is Krypton.