Answer:
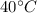
Step-by-step explanation:
Given
Coldwater is at
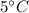
Hot water is at
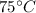
Suppose an equal amount of water is mixed i.e. m
So, the heat released by hot water is equal to the heat gained by cold water and the final common temperature be T
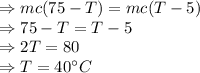
Option (2) is correct