Answer: The new pressure of the gas at this volume is 10 atm.
Step-by-step explanation:
Given:
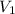 = 250 mL,
= 250 mL,
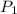 = 1.00 atm
= 1.00 atm
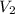 = 25.0 mL,
= 25.0 mL,
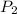 = ?
= ?
According to Boyle's law, at constant temperature the pressure of an ideal gas is inversely proportional to volume.
Therefore, formula used is as follows.
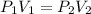
Substitute the values into above formula as follows.
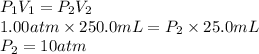
Thus, we can conclude that the new pressure of the gas at this volume is 10 atm.