Answer: There are 0.25 moles of NaOH in a sample that weighed 10g.
Step-by-step explanation:
Given: Mass of NaOH = 10 g
Molar mass of NaOH is 40 g/mol.
Number of moles is the mass of substance divided by its molar mass.
Therefore, moles of NaOH are calculated as follows.
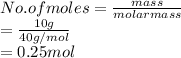
Thus, we can conclude that there are 0.25 moles of NaOH in a sample that weighed 10g.