Answer: There are
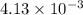 moles of Pb are contained in
moles of Pb are contained in
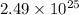 atoms Pb.
atoms Pb.
Step-by-step explanation:
According to the mole concept, 1 mole of every substance contains
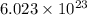 atoms.
atoms.
Hence, number of moles present in
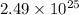 atoms of Pb are as follows.
atoms of Pb are as follows.
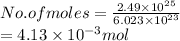
Thus, we can conclude that there are
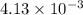 moles of Pb are contained in
moles of Pb are contained in
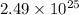 atoms Pb.
atoms Pb.